In the production process of communication plasma display screens (ACPDP), thick film printing technology has been widely used, which has the advantages of simple process, low cost, suitable for mass production, less material waste, and less pollution. However, the low precision of its printed graphics limits its further development, especially in the production of PDP boards with high transparency requirements. A wider display electrode can affect the luminous brightness of the PDP. This article focuses on substrate glass and proposes a method of surface modification using silane coupling agents to reduce its surface energy. Under the same conditions, the contact angle between the slurry and the glass decreases, making it difficult to expand on the glass surface. Ultimately, the printed electrode lines become narrower (still meeting the requirements of low resistance), improving the luminescence brightness.
1 Basic Principles
1.1 Introduction to Silane Silane is a special type of organic silicon compound, which can be represented by the general formula YRSiX3. It is a hybrid compound of organic and inorganic compounds, where X and Y are two groups with different reaction characteristics. X is prone to strong bonding with glass, SiO2 metal, etc. in inorganic materials (physical and chemical), while Y is prone to good bonding with resin, etc. in organic materials (physical and chemical). It can improve the actual bonding strength between polymers and inorganic materials, which may refer to the improvement of true bonding strength, wettability, rheological properties, and other operational properties. Table 1 shows some typical silane products available on the market. Chiye Organic Silicon Materials (Shanghai) Co., Ltd. has many uses for silane, and there are significant differences in different types. The main purpose of this work is to use it as a surface treatment agent for PDP substrate glass, in order to improve the surface performance of glass, reduce the surface tension of glass, control the wettability of slurry on glass, and thereby improve the precision of printing lines.
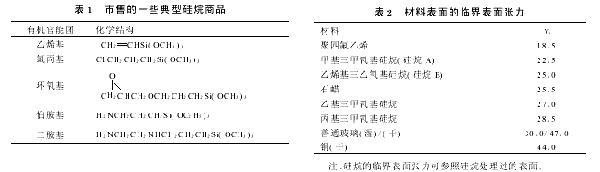
1.2 Theoretical Basis Table 2 shows the critical surface tension between some typical construction materials and partially silanized surfaces( γ c) From the values in the table, it can be seen that the values of silane A and B γ C is much lower than ordinary glass, and even more so than paraffin γ C is even smaller. If the surface tension of the substrate glass can be reduced, the contact angle between the slurry and the glass will decrease, the expansion effect of the slurry will weaken, and the printed electrode lines will become narrower. In reference [1], it was briefly mentioned that treating the glass surface with appropriate hydrolytic silane can control the wettability of the glass. But this is only a theoretical idea, and not all can achieve ideal results in practical applications. For a certain material, even the best coupling agent, if used improperly, will have little effect or even the opposite effect. The molecular orientation of the coupling agent and the physical properties of its thin film (solubility, solubility, and mechanical properties) controlled by the use method are equally important as the chemical properties of the selected silane.
2 Experimental Results and Analysis
2.1 Preparation of silane solution: Only a few organic trialkoxysilane can be immediately mixed with water, and most silane is insoluble in water before its alkoxy group (X) is hydrolyzed. The results of this experiment are shown in Table 3. Trialkoxysilane RSi (OR ′) 3 will gradually hydrolyze into corresponding silanols in water, which will eventually condense into siloxanes. The rate of these two reactions largely depends on the pH value of the aqueous medium. However, under the most suitable conditions, the hydrolysis reaction is relatively fast (a few minutes) and the condensation reaction is much slower (a few hours). Higher grade alkoxysilanes hydrolyze very slowly in water because they are highly hydrophobic. Both acids and bases can promote hydrolysis. In experiments, mixing silane B with acidified water and vigorously shaking it can dissolve organic trialkoxysilane in water and generate silanols, forming a clear and transparent solution. However, as silanol condenses into low degree siloxane alcohol, the solubility of the hydrolysis product containing silanetriol [RSi (OH) 3] decreases, and the clear aqueous solution of hydrolyzed silane gradually becomes turbid with increasing storage time, eventually precipitating in the form of oil droplets. From the experiment, it can be seen that the acidic aqueous solution of silane B is more stable, and the solubility and dissolution time vary with the pH value of the solution and the method of adding pure water. This work selected an acidic aqueous solution of silane with a concentration less than 1% and an ethanol solution to pretreat the substrate glass. The experimental results are compared as follows:
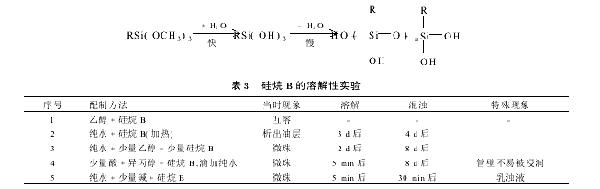
(1) The comparison of different solutions shows that the ethanol solution of silane B is more stable than the aqueous solution. However, due to the volatility of ethanol, the surface treatment time of the substrate with this solution is not too long. Moreover, compared with the same mass concentration of aqueous solution, the treatment effect of ethanol solution is slightly worse; (2) The comparison of different mass concentrations of aqueous solutions shows that silane B aqueous solutions with higher mass concentrations are not very stable. If the mass concentration is higher, they will not be soluble in water. A 1% mass concentration aqueous solution will become turbid within 10 days. In contrast, an acidic aqueous solution with a mass concentration of 0.5% has better stability and more obvious experimental results.
2.2 Reaction between silane coupling agent and glass surface. Silanol groups in silane solution play an important role in the coupling process. When low-quality concentration aqueous solution is applied to glass, a large number of silanol groups are oriented towards the glass. When dried at higher temperatures, silanols will highly condense into siloxanes (linear, cyclic, spherical). Polysiloxane can react with hydroxyl groups on the glass surface to form covalent (Si-O-Si -) - bonds. The surface silane structure of dry glass is shown in Figure 1, and the ideal silane structure of the glass surface after silane treatment is shown in Figure 2. Due to the weak intermolecular forces of polysiloxane, the surface tension of the treated glass is relatively low, which is beneficial for inhibiting the expansion of the slurry. In order to make the reaction between the coupling agent and the glass surface more complete, the time of silane treatment and the drying method after treatment are both important. The following are the descriptions: (1) The effect of different soaking times t on the surface treatment effect of silane coupling agent in the experiment. The main focus is to observe the improvement rate k of the printed pattern line width of the substrate glass before and after treatment, while also considering whether the operation is convenient and feasible. Figure 3 shows the influence curve of substrate glass soaking time t on line width improvement rate k in different solutions based on experimental results. Curve 1 shows the soaking result in a 1% mass concentration aqueous solution, and curve 2 shows the soaking result in a 2% mass concentration ethanol solution. From the curve in the graph, it can be seen that the treatment effect of ethanol solution is not as good as that of aqueous solution, and the volatile nature of ethanol solution also brings some difficulties to practical operation; A slightly longer soaking time in aqueous solution would be more beneficial, but from an efficiency perspective, it is advisable to keep the soaking time of the aqueous solution within 24 hours. In addition, it was also found during the experiment that using an aqueous solution of silane B with a long preparation time would weaken the effect, so the solution should generally not be left for more than 7 days. (2) The influence of drying method is due to the higher temperature, which is conducive to the reaction between silanol and glass surface in the coupling agent solution, reducing its surface tension, reducing the expansion of slurry on the substrate surface during thick film printing, and improving the improvement rate of pattern line width. Therefore, the substrate surface treated by high-temperature drying furnace after soaking is much better than that naturally dried at room temperature. Based on the experience of this work, selecting the appropriate temperature for high-temperature drying treatment is more effective than extending the soaking time at room temperature.
2.3 Performance indicators refer to the comparison between the part that has been immersed in silane solution and the other part that has not been immersed in silane on the same substrate glass. Therefore, the interference of various external conditions is relatively small, and the impact caused by different glass properties is also minimal. (1) The line width improvement rate involves soaking half of a substrate glass in a silane aqueous solution, while the other half is left untreated. The display electrode is then printed using conventional thick film technology, dried, and sintered. The results showed that the printed lines on the substrate glass treated with silane coupling agent had an average improvement rate of over 20% in line width compared to the lines on the untreated substrate. The electrode cross-section of the printed line is shown in Figure 4, where (a) is the electrode that has been immersed, and (b) is the electrode that has not been immersed. (2) After surface treatment of the resistance substrate, although the line width of the display electrode became thinner, the resistance did not increase but decreased. Due to the different electrode linewidths printed in the two cases, d1S2, the refined electrode actually has lower resistance. This not only meets the production requirements of display electrodes, but also helps to reduce the power consumption of PDP. (3) Due to the strong bonding between silane and organic compounds (organic solvents in the slurry), the wire breakage rate does not increase despite the thinning of printed electrode lines.
The best method for pre-treatment of substrate glass with silane coupling agent is to soak the glass in an acidic silane aqueous solution with a mass concentration of less than 1% for about ten hours (adding amine catalysts will have a better effect), and then dry it at a temperature of 150-200 ℃ for more than ten minutes. At this time, using the thick film process to print the display electrode of PDP, the line width improvement effect is the most significant, and the average improvement rate of the sintered line width reaches more than 20%. Moreover, this pre-treatment method is simple, cost-effective, and has a significant effect. The experimental results show that the method of treating glass surface with silane can indeed reduce the surface energy of glass, achieve the purpose of controlling wettability, reduce the obstruction of the display electrode on the effective luminous area, and thus improve the luminous brightness of PDP.
 add wechat
add wechat

24 hours tle021-58997516

Mobi13321881626
Copyright © 2002-2022 ChiYe (shanghai) Co,ltd Address:Shanghai in China Case Number:沪ICP备14048717号-1 Siemap